Dr. Roland Emmrich and Ralf Arnold
O.K.Tec Optik Keramik Technologie GmbH, Wildenbruchstr. 15, D - 07745 JENA, GERMANY
Poster presented on the occasion of the
"second PHARMANALYSIS Europe Conference and Table-Top Exhibition"
24-25 April 1995, Duesseldorf, Germany
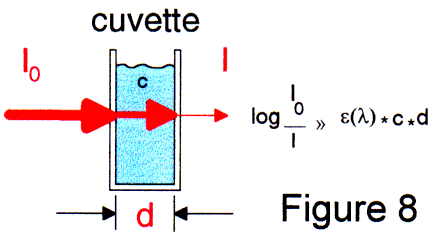
The traditional method to determine the release rate of active ingredients in drug formulations is to use spectrophotometers meters with flow-through cuvettes which have received samples that have been pumped into them from dissolution vessels a distance 1-2 m away. Since there is a delay involved in this step as well as in positioning each cuvette per dissolution vessel before the spectrometer, it is not possible to determine the short terms effects of dissolution process.
By using glass fiber cuvette dip probes, which are attached to the spectrometer, a realtime monitoring of the release rate is possible.
Glass fibers as light guides are known for more than 20 years. In the last few years, attempts were made to couple transmission cells with glass fibers to spectrometers.
The intent was to achieve fully automated monitoring, without the delays involved in obtaining samples from each vessel.
The dissolution apparatus consists of at least 2 dissolution vessels, and at maximum of 6-7. A fully automated system would therefore multiplex a maximum of 7 vessels, or locate a fiber probe in each vessel.
Today, there is no glass fiber based dissolution monitoring system on the market. We will thus evaluate fiber probe designs and the major problems in coupling fibers to cuvettes and spectrometers.
The glass fiber technology has been spurred by the needs of the telecommunication industry. Specific transmitting "windows" are required for light pulses from lasers at 630 nm, 820 nm, 1060 nm, 1320 nm. These fibers are monomode fibers. For most sensor and spectrophotometric applications multimode fibers are necessary.
Glass fiber manufacturer optimize the glass for the lowest attenuation at these wavelength windows. Additionally the glass fiber attenuation depends from the purity of the glass preform. A preform is a special glass ingot which is prepared to pull out the fibers.
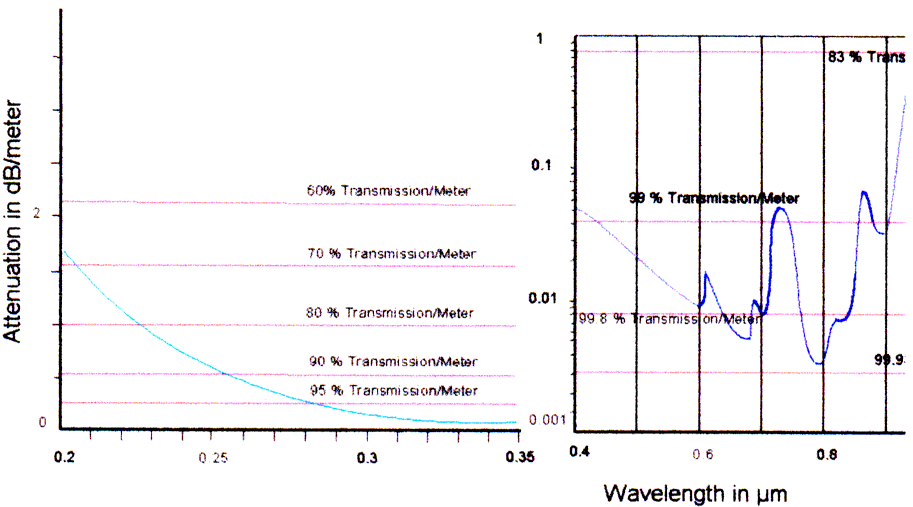
A typical attenuation curve of fused silica material is shown in Fig 1.
Figure 1
We recognize an increase in attenuation, especially in the UV. In the 200-350 nm range, the transmission per meter drops to 60% or less!
Transmission data from the glass fiber manufacturer are based on the data from the preform ingot, i.e. the transmission of real glass fiber may be even smaller.
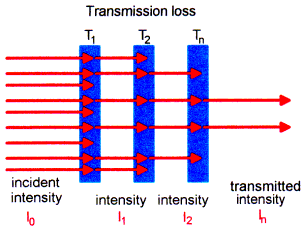
Figure 2
Example: If the path through the glass is 1 m and the transmission at a selected wavelength is 90% we get:
If we now have a 2 m path we will get only 81 %:
For a real application we estimate that 2 m fiber in each direction (2 m from light source to the probe and 2 m back to the detector) are necessary. So we will transmit only 65%! In the UV, where the transmission per meter is less than 70%, we can transmit less than 24%!!! This is one of the important limitations of using fibers for sensor and spectrophotometric applications.
A second limitation is the NUMERICAL APERTURE.
![]()
The light comes out of the multimode fiber in a cone shape (not collimated). Now, it is extremely important to couple as much light as possible into the fibers, or to use optical devices such as lenses or mirrors.
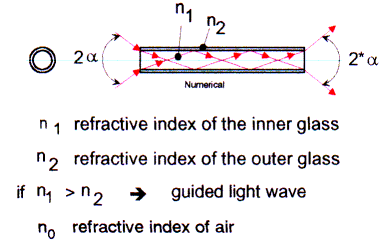
Figure 3
So called acceptance angle 2a determines the F/ number. Light which is outside the acceptance angle does not enter the fiber!
The conclusion is that these losses in a fiber probe system are probably higher than the estimated 80 % in the example above.
At the beginning, we made a simple experiment as shown in Fig 4. One fiber emits light with a constant intensity I0 in the direction of the plane mirror. A second fiber collects the reflected intensity and guides it to a detector.
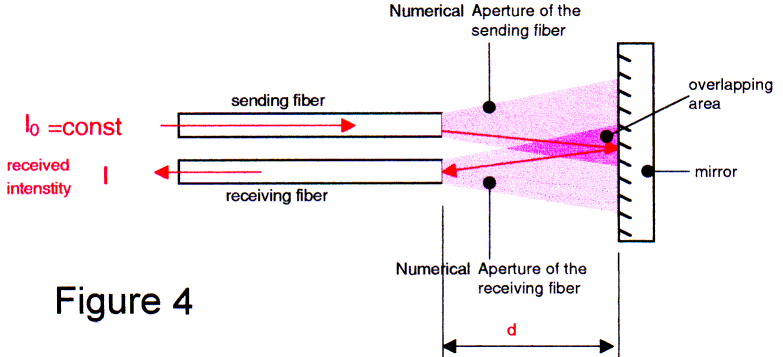
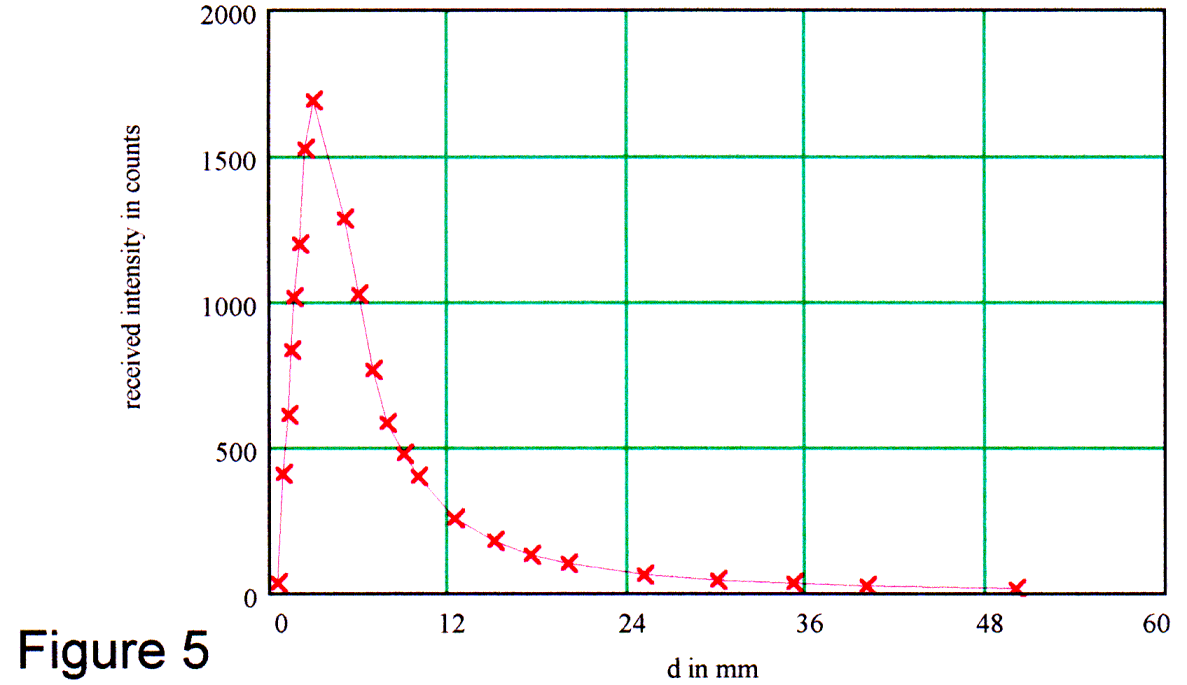
In Figure 5, we show the rapid drop in the received intensity when we increased the distance between fibers and the mirror.
To increase the received intensity, various optical setups are reported in the literature.
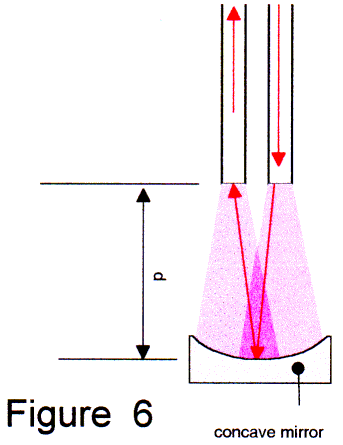
One possibility (Fig. 6) is to use a concave mirror. The disadvantage is "flow sink effect'. Particles will sediment during the dissolution process and obstruct the reflection over time. However, by using a plano-convex lens where the convex side is coated with a excellent UV-VIS reflecting layer (aluminum, silver), we can get a plane surface without any sedimentation.
The principles of Fig 4 and Fig 6 could be used for a cuvette design. However, a third problem is encountered: straylight generated in the fiber probe design.
The fibers are made by high purity fused silica glass at the core, and a second glass or polymer with different refractive index as the cladding (see Fig 3).
Water and all water based liquids are very aggressive to the silicon-oxygen-tetrahedrons of the fused silica [1]. So a layer of Si-OH-groups is covered with a layer of H20-molecules. In these layers, all ingredients which were in contact with the fiber core can be compound. The degree of water corrosion depends on the pH. Since the pH is not constant in a dissolution process, the optical transmission will changing. Also, cleaning the output and input end of a 400 or 600 pm fiber is too difficult. For fast cleaning and a durable system, the simplest way to protect a fiber is to use a regular fused silica window.
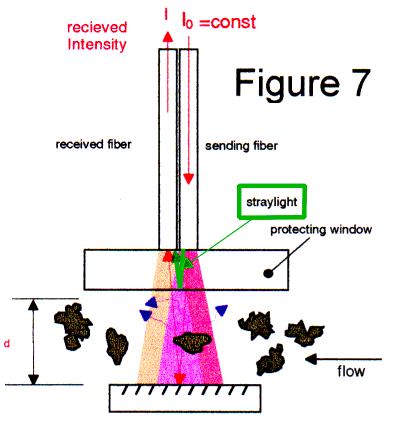
At the inner surface between the protecting window and the liquid, straylight that is caused by the diagonally striped incident light from the light cone of the sending fiber can be avoid. In spectrophotometric measurements, the ratio of the sent and received intensities is calculated.
The generated straylight thus limits the dynamic range up to 0.7 ... 0.8 absorbance units. With tricks we can get approximately 1.5 AU. Also, the diagonally striped light produces nonlinear results if particles cause turbidity.
In on-line measurements, no dilution and filtering is possible using a dipping fiber probe!
We can then sometimes expect a sharp rise in concentrations and the optical density, and a undetermined flow of particles of different sizes.
With the so called "transflection" probes (Fig 4, Fig 7), we have small dynamic range and the determination of turbidity in a spectrum is uncertain.
Only a "TRUE CUVETTE" probe can achieve dynamic range up to 3 AU and determine turbidity.
A true cuvette system can be made by a coaxial, face to face position of sending and receiving fiber or fibber bundles [2]. Because of divergent light cone and the acceptance angle, only small paths are possible. Compared with a regular 10 mm cuvette path, the signal to noise ratio is extremely bad [2].
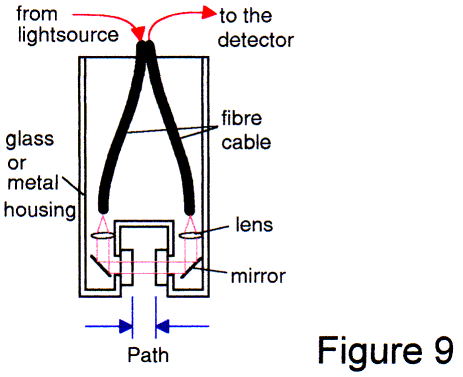
A more expensive arrangement is shown in Fig 9. Such designs are being marketed today. The performance is excellent. The only disadvantage is the large size and/or large diameter. The lenses/mirrors/prism require space. For a 2-3 mm diameter light beam, a space of 5-8 mm is required inside the housing. When you include the path and the walls, the diameter of such a probe would be at least 40 mm. For dissolution applications this design is not suitable.
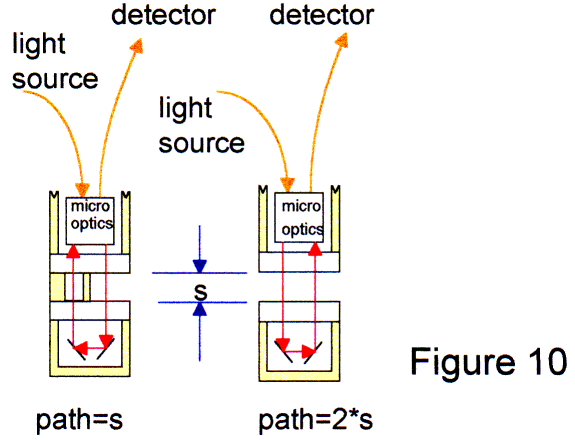
|
The probes contain a microoptical system to collimate the light cone from sending fiber. The small light beam with a diameter at least 1.5 mm traverses the liquid path and is reflected by a special mirror system. The microoptics shape the received light and direct into the acceptance angle of the receiving fiber to get a maximum throughput. Because of the almost collimated light, paths up to 50 mm and in part more are available. Also, the signal to noise ratio is much better than a "transflection" system. These new true cuvette system is available in size of a 12 mm diameter dipper: the same size like a pH-electrode. The performance is excellent. For example in Fig 11-13, the presence of acetone in the ppm-level is shown at the 260 nm point. The probe was coupled with a new type of CCD-spectrometer built into a PC. The shown resolution is 32 nm. The measurement show the performance of a first 50 mm path fiber probe system. |
With traditional spectrometers having a sample compartment it is not possible to use different length of fibers. The spectrometers have a light source and a dynamic range for air path. With the best available sample compartment to fiber adapter we found 50% losses to couple light from the monochromator output into the acceptance angle of the fiber and shape the output aperture to direct the divergent beam to the detector. However, every spectrometer with sample compartment has a different optical construction. Optimization of such adapters is difficult and because of the constant light power and the high losses at the adapter in double beam spectrometers a beam absorber with 1% transmission in the second beam is necessary to get a balance of the two detector channels.
By the need of the process monitoring in chemical industry a couple of pure fiber coupled spectrometers for UV-VIS and NIR were developed. The manufactures of these spectrometers consider the high attenuation of fiber arrangements in the UV. With spectrometers which are designed for fiber coupling we get an excellent signal to noise ratio.
For our experiments we use a small CCD spectrometer. The original setup of the optical bench was created by a small American company. We made a redesign to increase the stability and wavelength accuracy. By using 12 bit ADC-boards a powerful small spectrometer system can be built in small rack, a stand alone housing or a PC-housing (Fig 14-16).
Xenon flash, Deuterium and Tungsten-Halogen light sources are available in disk storage rack size.
The optical and the electronical setup of both designs is equal.
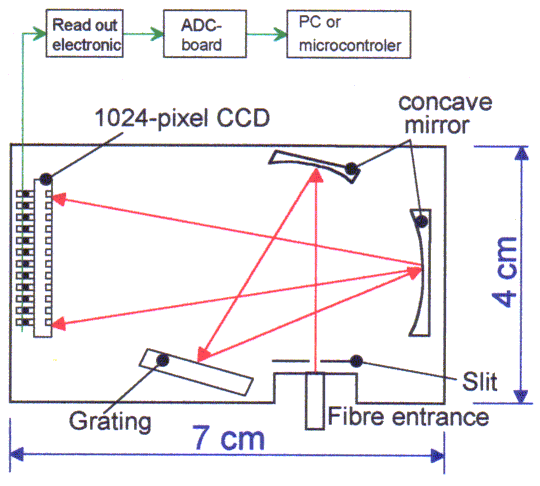
Miniature CCD spectrometer module
Figure 14
Figure 15
Two spectrometers and the read out electronic are build in a rack. The light source is build in a separate and shielded slot of the rack.
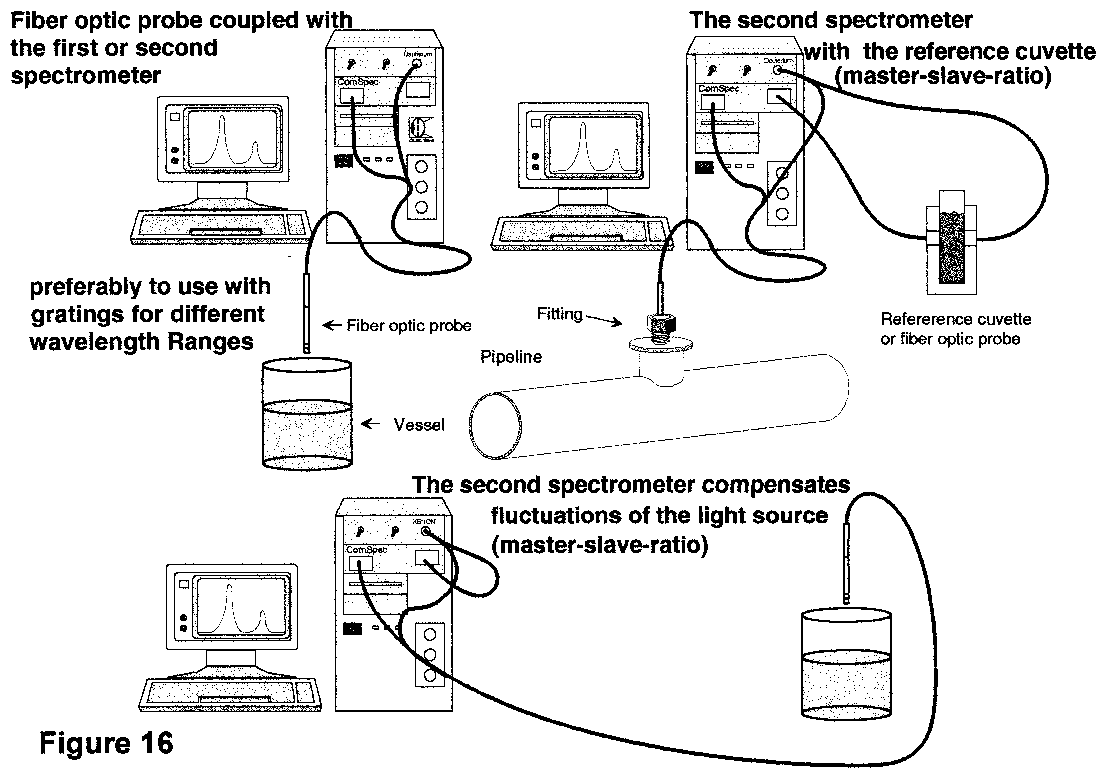
A real advantage of the CCD spectrometer is the possibility to run 2 spectrometers simultaneously with the same grating and in the same wavelength range or like a tandem with different wavelength ranges.
This allows a powerful customized setup of the spectrometer arrangement even for dissolution monitoring.
The software includes options for changing the integration time, number of flashes, pixel resolution and a gain to get the best signal to noise ratio. During running in realtime the scope mode shows the intensity to optimize the setup.
Furthermore absorbance, transmission and irradiance can be selected. All data can be saved in ASCII-files.
Two important options are available which are interesting for release rate monitoring.
With the option "kinetic sampling" the spectrometer samples the spectra and shows the spectra after sampling (Fig 17).
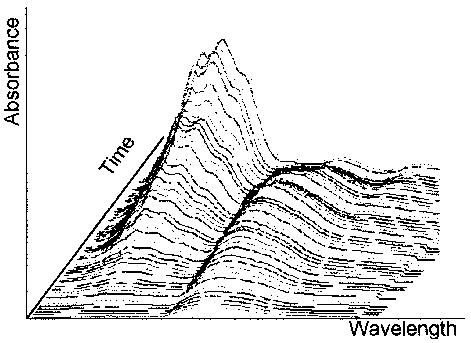
Figure 17
In a realtime dissolution process we find a lot of particles in different sizes. So we will get a base line shift over time. The elimination in the kinetic mode is difficult.
Two methods are described in the literature; one method is the derivative spectroscopy. With the second derivation the elimination of turbidity background is possible [3], However, we need the 2. derivation in realtime! Therefore we need a powerful PC to handle more than 4 data files 1100 data each in realtime. This is hard to do with the DOS limitation at 640 kB or a Windows Software which inquires DMA.
A much more simple way is using the process recorder software option. With this option the size of the calculated data files is smaller. Four different recorder channels with monitor-scroll in colors red, green, blue and yellow can be activated. With wavelength choice and programmable mathematical operations (e.g. subtraction of two absorbance values from different wavelength) a turbidity compensation is extremely simple.
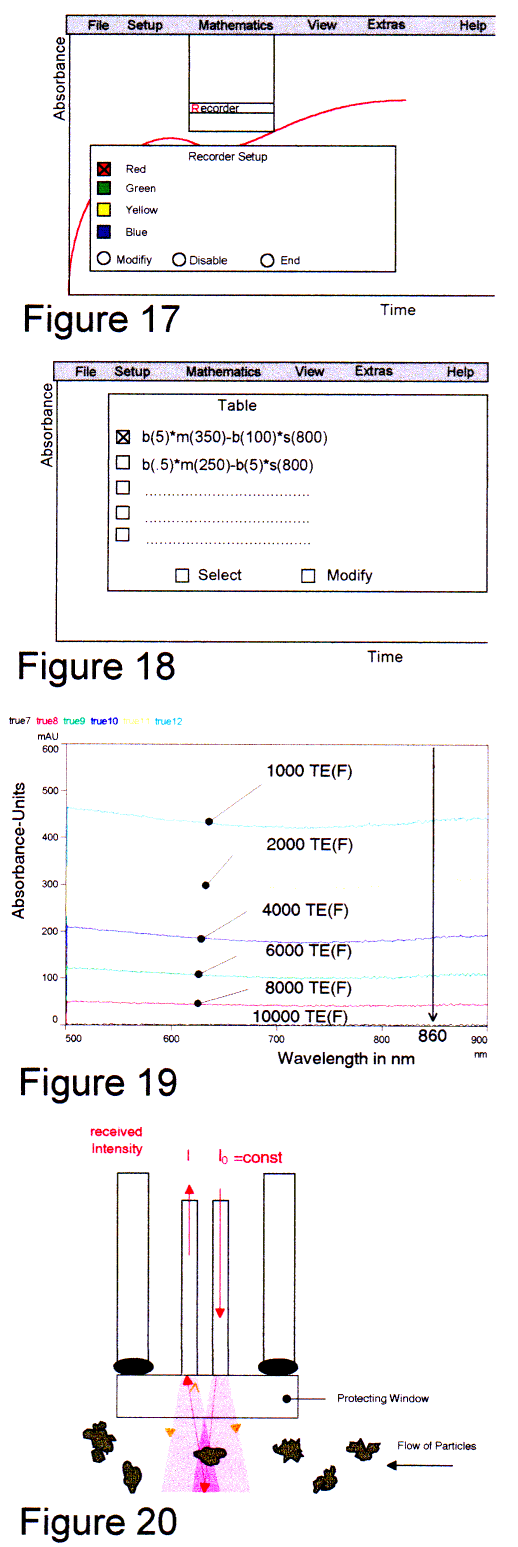 |
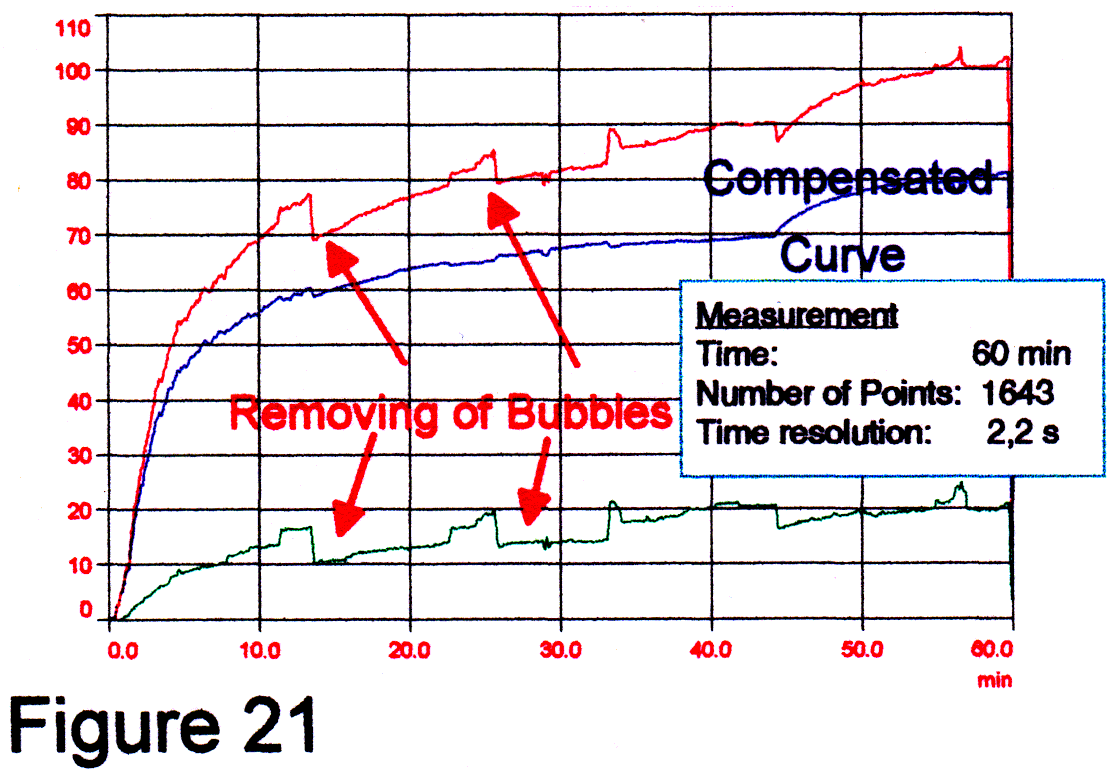
Example Fig 18-19: The shown red line represents the absorbance value at 350 nm (with 5 nm averaging of the master channel) extract the absorbance value of the slave spectrometer at 800 nm with 100 nm averaging. A second lane is not activated. Between 500 - 900 nm we found a very small dependence of the absorbance caused by particles from the wavelength. These measurements we did with Formacine standards described in DIN and ISO dossier [4], a CCD-spectrometer and a fiber probe shown in Fig 20 (backscattering principle). RESULTSNow, if we know where the drugs absorb in the UV we can distinguish the drug caused absorbance from the particle caused absorbance. With the powerful small spectrometer, the "true cuvette" probes and the process recorder software we attempt real release rate monitoring. We used a SOTAX 6 dissolution tester. All data were scaled for each spectrum to a certain mixture ratio (in the enclosed example 1:25 of the ESIDREX drug). During the measurements a sediment of small particles and gas bubbles was found, so that bubbles had to be removed time after time Fig 25. These bubbles act as small lenses. Using Ceramic-probes this phenomenon was never observed. The reason is the hydrophobic surface of ceramics and sapphire. By briefly shaking the metal made probes, the bubbles were removed. This is seen as a "zig-zag" on the output curve. The turbidity compensated graph "esi2hc3" (Fig. 21) shows the real release rate of the drug. The influence of turbidity is minimal. With ceramic made probes the influence should be zero. We used the enclosed setup. |
 |
Spectrometer: ComSpec2X Fiber-probe: TS-EM2/UV Drug (Esidrex): Courtesy CIBA-GEIGY |
With the process recorder and a "true cuvette" fiber probe system realtime release rate monitoring is feasible. In a next step a design of dissolution monitor for 6-7 vessels get closer.
[1] H.H. Dunken: Physikalische Chemie der Glasoberflaeche, Deutscher Verlag fuer Grundstoffindustrie, 1981
[2] Chi-Shi Chen, C.W. Brown, Pharmaceutical Research, Vol. 11, No. 7, 979-983(1994)
[3] Birkhaeuser: Laborpraxis, Bd. 4 Analytsiche Methoden, 4. ueberarb. Auflage, Basel-1990
[4] DIN 38404/ISO 7027